Published on the 7th March 2022 by ANSTO Staff
Key Points
-
Some good news for the management of nuclear waste and nuclear fuel with the identification of a stable form of technetium oxide
-
In another publication, Investigators from ANSTO and Australian and international collaborators elucidated the mechanism by which potential nuclear waste forms respond to irradiation
-
Specialist techniques at ANSTO for the study of nuclear waste we re used to confirm the findings of both studies
ANSTO researchers have demonstrated longstanding expertise in the study of radioactive waste, nuclear fuel and nuclear waste forms with two recent journal articles in a special issue of Frontiers of Chemistry on ‘Ordered and Disordered Cubic Systems: Pyrochlore to Fluorite, Now and on the Horizon’.
Prof Gordon Thorogood, a co-author on the two publications, was also a guest editor of the special issue with American and French experts.
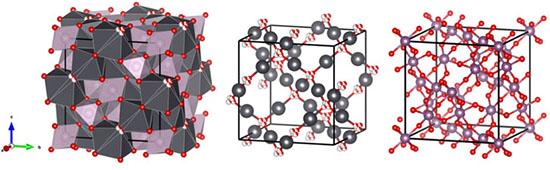
In a paper from Prof Brendan Kennedy of the University of Sydney and his associates, including Prof Gordon Thorogood and others at ANSTO in Frontiers in Chemistry, the authors provide the first report of a stable valence 5 technetium oxide.
The lead technetium pyrochlore investigated in the research is found in nuclear fuel and nuclear waste.
The migration of 99Tc is a significant challenge in nuclear waste management.
"As well as the fact that no stable valence 5+ oxide of technetium exists, no one previously has been able to make technetium oxide with a stable valence state,” said Prof Thorogood.
”People made the assumption that if you had 5+ technetium oxides, they would break down into 4+ which is stable and then 7+ which is unstable.
“However, if a stable 5+ technetium oxide is formed in a nuclear waste form, it is probably good news,” said Prof Thorogood.
There was some confusion previously as to whether the compound was a pyrochlore or perovskite. Some of the perovskite chemistry reported a decade ago that was first noticed by Prof Kennedy, seemed inconsistent with a pyrochlore structure.
Thorogood and Kennedy and associates decided to investigate in an effort that took a number of years.
The team combined synchrotron powder diffraction and Neutron powder diffraction to determine the structure of the lead-technetium pyrochlore Pb2Tc2O7-d, X-ray absorption spectroscopy to confirm the valence state was greater than 4+ and Raman spectroscopy to confirm a change in local structure and coordination of the ions.
They observed a move from order to disorder by the Pb cations and O2 anions, which was meaningful.
“The disorder appeared to allow for larger bond lengths for the technetium, resulting in it being in the higher valence state,” said Prof Thorogood.
The unique facilities at ANSTO that are designed for the handling of radioactive materials, were the only place that the research could be undertaken in Australia.
“Not only do we have the infrastructure that allows us to look work on radioactive materials, we also have the knowledge to make the materials safe so that they can be studied off-site as well.”
ANSTO technical specialist Kevin Thorogood built a special sample holder to contain the material for safe handling during the Raman spectroscopy measurements at the UNSW.
Other ANSTO scientists contributed to the study, including Dr Max Avdeev, Dr Zhaoming Zhang, Dr Hanliang Zhu and former staff member Dr Kia Wallwork.
DOI: https://doi.org/10.3389/fchem.2021.706269
In a second paper also in Frontiers in Chemistry, co-authored by Prof Thorogood with associates from the University of California Irvine (US),CEA, HZB, and Nagaoka University of Technology (JAPAN), the investigators elucidated the mechanism by which titanate and zirconate pyrochlores, potential nuclear waste forms, respond to irradiation.
Under irradiation, Er2Ti2O7 had been routinely reported to transform to amorphous material and Nd2Zr2O7 to transform to defect fluorite.
“But there was little clarity on the cause and progress of transformations,” explained Prof Thorogood.
The international team of researchers used helium ion irradiation (approximately 1 displacement per atom) at the near-surface of the pyrochlore as a form of accelerated radiation damage to simulate the effects caused by either fission fragments or the interactions with neutrons.
The irradiations can be routinely done at ANSTO’s Centre for Accelerator Science.
Using samples only 100 microns thick, combined characterisation methods included neutron diffraction, electron backscatter diffraction (EBSD) and TEM, to gain mechanistic insights into how the disorder process proceeds.
Neutron diffraction data on the Echidna instrument at ANSTO’s Australian Centre for Neutron Scattering and Synchrotron X-ray powder diffraction (SXRD) was performed at room temperature on the damaged samples to determine the difference in disorder and the behaviour of the cations vs. the anions.
They also wanted to compare the degree of stress and strain in the damaged material with the undamaged material.
Neither the Er2Ti2O7 pyrochlore nor the Nd2Zr2O7 pyrochlore underwent a phase transformation to defect fluorite when they were irradiated.
There was evidence of hydrostatic stress in the Nd2Zr2O7 sample, while approximately 30% of the Er2Ti2O7 sample became amorphous.
Neutron diffraction indicated the sample was made up of two phases.
"We concluded that one phase was the undamaged region and the other phase was the damaged region.," said Prof Thorogood.
“What we think happened is that the irradiation has created residual stress in the surface that has moved like a stress front into the ceramic.
“It is likely that the sample builds up residual stress and then breaks up into smaller and smaller pieces forming tiny domains,” said Prof Thorogood.
ANSTO scientists who also contributed to this study included Prof Mihail Ionescu, Dr Daniel Oldfield, Joel Davis, Dr Gregory Lumpkin, and Dr Max Avdeev.
DOI: https://doi.org/10.3389/fchem.2021.706736
In an editorial the editors described the goal of a special edition was to provide an overview of what has traditionally been thought to be disorder in the pyrochlores, how it relates to the formation of a defect fluorite structure, to highlight the latest discoveries as well as the path forward in this research area.
The versatility of these structures has led to their application in all manner of uses, ranging from fuel in a nuclear reactor, fuel cell anodes, ion conductors, nuclear waste immobilization, thermal barrier coatings, catalysts, and superconductors among others.











